Making improvements to a simple experiment on combustion.
C. G. Hodgkin
Lecturer in Science Education, Edith Cowan University
Mount Lawley, Western Australia.
Posted with permission of the author. This paper was published in the Australian Science Teachers Journal, March 1995, Vol. 41, No 1, p. 47.
Abstract
In this paper the author examines a conventional science "experiment" and suggests how a new approach can help students to develop concepts and process skills. He suggests that inaccurate science and conventional wisdom are often not challenged by traditional experimental procedures, but that a constructivist approach may help students to reconstruct their ideas in a more scientific frame.
Some familiar, (dare I say "traditional"), science experiments seem to lose all meaning when one stops to look at (a) the science, and (b) the cognitive development which is supposed to be evident. One such "experiment" arises from the following problem.
Problem: "When you light a candle in a closed jar, why does the flame go out?"
The traditional, simple, approach to this experiment is to set a candle into a plasticine mat, light it, and place a jar over the top. In a few minutes, the candle goes out making a cloud of smoke in the jar.
If you discuss this experiment with most school students - being careful not to inhibit their opportunities to express their prejudices and opinions - two possible hypotheses arise.
1. "The flame uses up a gas (oxygen?) as it burns and when all this gas is gone, the flame goes out."
(More accurately this may be expressed as: "The flame uses up oxygen as it burns and when enough has been used up (not necessarily all), the flame goes out.")
2. "The flame produces a gas (carbon dioxide?) which won't burn and when enough has been produced, it puts the flame out."
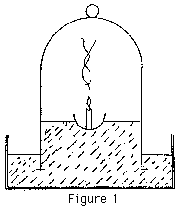
The problem is how to test these hypotheses in any scientifically defensible way. One very usual method of doing this experiment is to float the candle on a little dish in a water trough and lower a jar over it (Figure 1). If you do this smartly enough, the candle will burn for a little, go out, and water will rise in the jar to about one fifth of the height, thus "proving" that the candle dies when it has used up the 20% of oxygen in the air. This is rubbish!
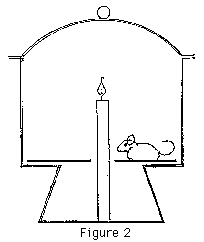
Take a large jar, (a desiccator will do well), fix a tall candle in the bottom and put a mouse in the jar (Figure 2). Light the candle and seal the jar. The mouse will NOT die. In fact it will probably not even be a bit distressed. The candle will, however, go out in the usual way. Clearly there must be a fairly minimal gas change in the atmosphere. (Actually, the candle probably "drowns" in the hot CO2 in the top of the jar, which the mouse is too low down to experience.) This simple demonstration will, it is hoped, create some cognitive conflict. (Nussbaum & Novick, 1981)
Consider this most significant error. If the jar is not sealed, then it isn't possible to be sure that some of the gas in the jar escaped from the jar during the experiment, or that some more air entered the jar during the experiment.
At this stage, the teacher will need to stimulate students into thinking through the experimental procedure. This is a model described well by Dawson (1992). The following might be the path for students to be helped to generate.
A. We know that the gas in the jar will be heated by the burning candle so there is a good chance that any changes in volume could be caused by this. If we want to investigate changes in gas composition, an accurate test will require us to test gas from a sealed jar to see if it contains the same mix of gases after the experiment as before. It would be a good start to look for volume changes first. These will have to be measured on the cooled gas in the jar to be sure that they are not simply due to heat expansion.
B. The experiment should use a large screw cap jar. A small candle can be fixed to the inside of the jar lid, which is placed on the desk. Once the candle is lit, the jar can be quickly placed over the candle and the lid screwed on as quickly as possible. It must be screwed airtight. After the candle goes out, the jar must be left to cool for several minutes until it has returned to room temperature. Then it can be lowered into a large bucket of water and the cap gently loosened until either escapes gas or water enters the jar. This experiment will hopefully identify that there are minimal changes to the volume. (Almost certainly there will be some, but not anywhere near 20%). Therefore, the burning candle uses AND produces gas.
Having established this idea, students need to be encouraged to think of procedures to test the gases in the jar. One presumes that they know that CO2 is uniquely determined by its effect on lime water - an easy qualitative test.
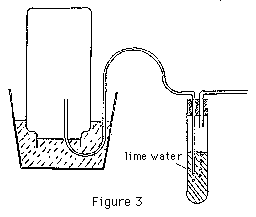
C. To make this test, the gas must be drawn out of the jar through lime water. A suction system and a bubbler will be needed (Figure 3). This should demonstrate that there is a significant amount of carbon dioxide in the gas. (Students should test to be sure that there is more CO2 than was in the air to begin with.) The logical inference is that at least some of the air in the jar has been converted into carbon dioxide. If the jar from the volume experiment is left sitting in the bucket of water, the CO2 will likely dissolve in the water fairly quickly and changes in volume will now be observed.
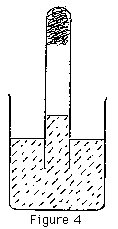
D. The percentage of oxygen present in a quantity of air can best be measured by using wet steel wool. The steel wool should be washed with methylated spirit to remove grease, rinsed with water and pushed into the bottom of a large test tube. The test tube can be filled with gas from the jar and left up ended over water. (As above, students need to test ordinary air as well.) In 24 hours (approx.) all the remaining oxygen will be combined with the steel (Figure 4). Whatever is left in the test tube is not oxygen. (Note that this is a fairly effective way of finding the percentage of oxygen in air.)
At this stage students ought to be able to make sensible explanations of what is happening to the air when a candle burns on the basis of some interpreted data.
We still haven't decided whether it is the lack of oxygen or the excess of CO2 that causes the flame to go out. In fact, as is mentioned above, the dousing of the candle by CO2 may be the causative factor but not in terms of the total quantity of gas in the jar, but merely the percentage of CO2 in the gas around the flame of the candle. However, students are now in a position to propose experimental procedures which would remove CO2 from the jar as fast as it is formed and find out if the flame burns for longer. They might also suggest the use of artificial gas mixtures, such as CO2 enriched air.
The teacher might suggest to students that they can explore the problem further by finding out if the size of the candle makes any difference. This will provide more opportunities for careful data gathering to investigate their hypotheses.
This kind of thinking through activity needs to arise in a guided interaction sequence. The use of this procedure encourages students to construct reality for themselves. (Driver & Oldham, 1986). It provides an opportunity for them to develop their skills of experimental design. It also provides an experience in which they test and reconstruct conventional wisdom.
REFERENCES.
Dawson, C. (1992). The Scientific and the Everyday: Two different ways of knowing. Some implications for Science teachers. Australian Science Teachers Journal. 38,1,19-24.
Driver, R. & Oldham, V (1986). A Constructivist Approach to Curriculum Development in Science. Studies in Science Education. 13,105-122.
Nussbaum, J & Novick, S. (1981). Creating Cognitive Dissonance Between Students' Preconceptions to Encourage Individual Cognitive Accommodation and a Group Cooperative Construction of a Scientific Model. Paper presented at the Annual Convention of the American Educational Research Association held in Los Angeles, California.